Gut Reaction
Understanding how infection causes stomach cancer could lead to new treatments and prevention
June 17, 2010 | Leigh MacMillan
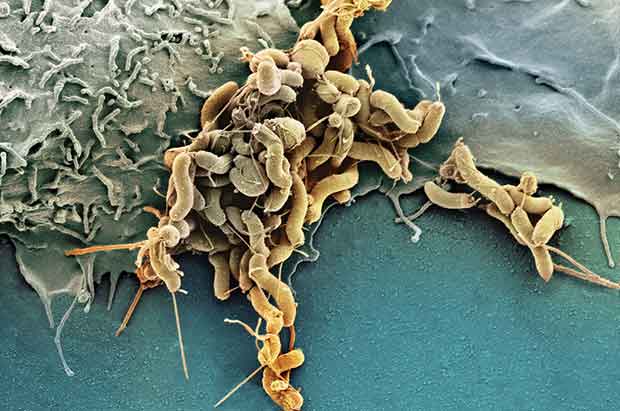
Richard Peek, M.D., laughs at the memory of his introduction to Helicobacter pylori – a twisted sausage-shaped bacterium that takes up residence in the human stomach.
He was a medical student at the time – around 1987 – and was following a patient with a bleeding ulcer. The patient had an endoscopy procedure to view and take biopsy samples of the stomach and intestinal tissue.
“I went to his room afterwards, and he told me that the doctor said there were ‘helicopters’ in his stomach,” Peek recalls.
Peek didn’t know much about ‘helicopters’ then, but in the years since, the bacterium that causes stomach ulcers and is the major risk factor for stomach cancer has become the focus of his research.
By studying Helicobacter pylori (H. pylori) and stomach cancer, Peek and his colleagues hope to unlock the secrets of how infectious agents cause cancer and find ways to prevent it.
“If we can intervene early on – to eliminate the chronic inflammatory process caused by infection – then the chances of reducing the cancer risk are much higher than if we treat later after pre-malignant changes have already occurred,” says Peek, the Mina Cobb Wallace Professor of Gastroenterology and Cancer Biology at Vanderbilt University.
Cancer germs
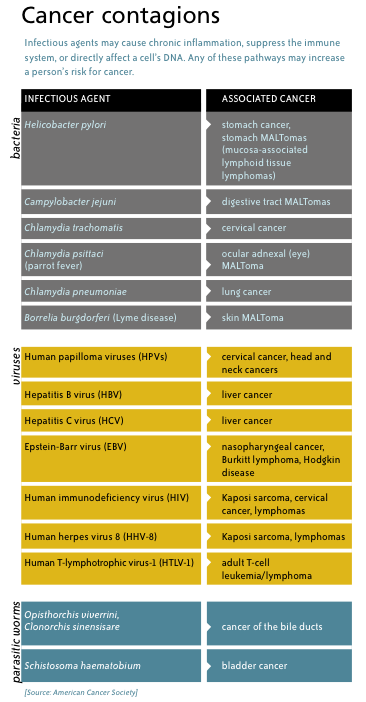
H. pylori is an infectious agent – a bacterium – that has been linked to an increased risk of cancer. It’s not alone. Other bacteria, viruses and parasites are associated with a variety of cancers. Current evidence suggests that as many as one in five cancers worldwide may have an infectious cause.
Viruses can insert their own genes into a cell’s DNA and disrupt the genes that regulate cell division and death. In the case of bacteria and parasites, one of the main problems appears to be chronic inflammation – the body makes an attempt to eliminate the pathogen, but it fails.
“Many of these organisms have developed strategies that allow them to persist or smolder in their hosts for decades,” Peek says. “That’s a common theme that we’re seeing that distinguishes infectious agents that cause cancer from those that don’t.”
It’s possible, he adds, that even more cancers will be linked to infectious agents as technology enables the identification of previously unrecognized bacterial and viral populations.

“Whether or not every inflammation-associated malignancy will have an infectious cause, I can’t say, but I do think that we’re going to be discovering more infectious agents that cause malignancy.” –Richard Peek, M.D.
“Whether or not every inflammation-associated malignancy will have an infectious cause, I can’t say, but I do think that we’re going to be discovering more infectious agents that cause malignancy,” Peek says.
Evolutionary biologist Paul Ewald, Ph.D., of the University of Louisville, thinks we’ll ultimately learn that infections cause most human cancers.
“If I were going to put my money on it, I would bet that by 2050 – hopefully earlier – we’ll have found that more than 80 percent of all human cancer is caused by infection. The number could be as high as 95 percent. In 1975 it was considered to be zero,” Ewald said in an interview published last fall in Discover magazine.
If infectious agents cause cancer, then preventing infection in the first place should provide a sure-fire way to prevent cancer. That’s the goal of vaccines like those directed against human papilloma virus, a major cause of cervical cancer, and hepatitis B virus, a cause of liver cancer.
But there aren’t vaccines for every infectious agent linked to cancer, and even if there were, they would be unlikely to reach every person at risk. So treatments are needed to eliminate the smoldering infections.
In the case of H. pylori, antibiotics can eliminate the infection. The problem is figuring out who should be treated. Half of the world’s population is infected with H. pylori, yet only about 1 percent of infected individuals will develop stomach cancer. And the bug may even be conferring beneficial effects to some of its hosts.
“I think there should not be widespread policies of testing and treating individuals for many of these organisms because only a minority of people who are infected have adverse outcomes,” Peek says. “For us that justifies trying to understand how the bacteria really causes cancer and who is most at risk. Then we can focus treatment efforts on those small pockets of high risk populations.”
Colombian clues
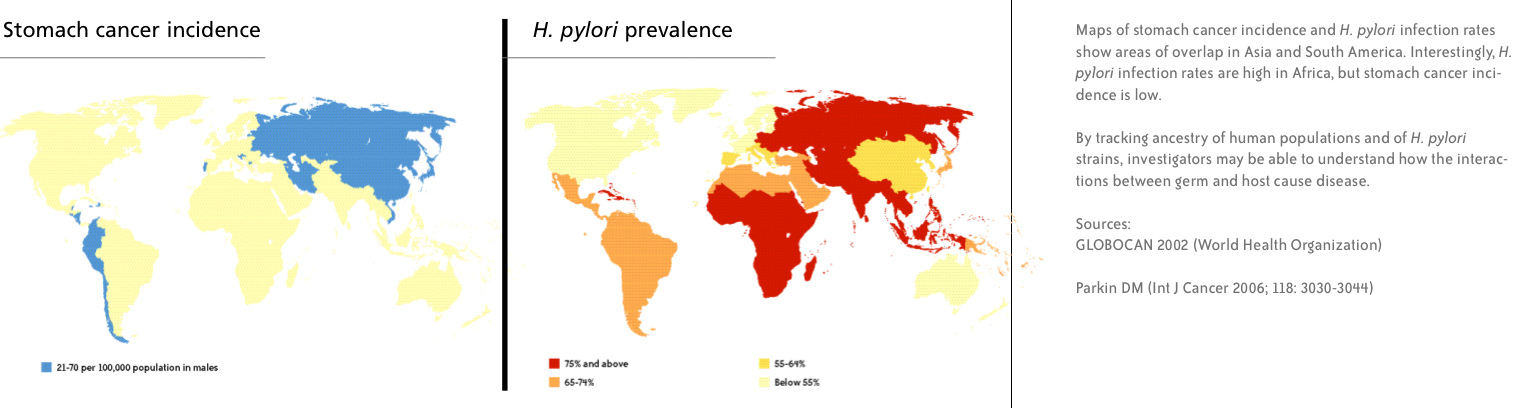
Stomach cancer is the second leading cause of cancer-related deaths worldwide (lung cancer is first). The highest rates of new stomach cancers are in Japan, throughout Asia, and in parts of South America.

Pelayo Correa, M.D., thinks a “perfect storm” of genetic and environmental factors is responsible for the high risk of stomach cancer in the mountains of Colombia. “…It’s the bug and the genetics of the person and the diet all together.”
One pocket of increased stomach cancer risk sits high in the Andes Mountains of Colombia.
As a new physician, Colombian native Pelayo Correa, M.D., began to compile a cancer registry in the city of Cali, Colombia, where he was practicing. He found that stomach cancer dominated the list, and he noticed that people who had come to Cali from the Andes in the southern part of the country had a higher incidence of stomach cancer than those from other parts of Colombia.
“So we went there to the mountains – the first time was in 1964 – and we started doing a series of studies which are still going on today,” says Correa, the Anne Potter Wilson Distinguished Professor in Colon Cancer at Vanderbilt. “We have learned many things with that population.”
In the early years, Correa and his colleagues examined biopsy samples and characterized the progression of gastric lesions – a cascade-like process that begins with inflammation of the stomach’s mucosal lining (gastritis) and proceeds stepwise to cancer. They estimated the rate of transition for lesions, and they sought factors that were contributing to the region’s high cancer rate.
They didn’t consider H. pylori – because it hadn’t yet been identified. The bug and its link to gastric ulcers were discovered in the 1980s, and in 1994 the International Agency for Research on Cancer classified H. pylori as a group 1 carcinogen – an agent that is carcinogenic to humans.
“There’s no question that what drives the carcinogenic process is the infection of this bug,” Correa says. “But it’s modulated. Very few people will get cancer.”
But who will get cancer? And can these people be identified and treated to prevent the cancer from developing?
Correa knew that people in the coastal regions of Colombia had very low rates of stomach cancer, and he saw a golden opportunity to compare the two populations in order to understand what puts the mountain people at high risk.
The investigators quickly learned that H. pylori infection alone wasn’t the culprit.
“The risk of cancer in the mountains is 25 times higher than at the coast, but the infection with the bug is the same – about 80 percent of people are infected,” Correa says. “It’s much more complicated than infection alone.”
The two populations have provided a rich resource for Correa and his collaborators. Over the years, the investigators have discovered that differences in the types of H. pylori (“strains”) that infect the two groups, how a person’s immune system responds to the infection, and environmental factors like diet and co-infection with parasites all contribute to the varied risk for stomach cancer.
“It looks like it’s multi-factorial – it’s the bug and the genetics of the person and the diet all together,” Correa says. “And in the mountains you have the perfect storm – the worst bugs, the most susceptible hosts, and a diet high in salt and low in fruits and vegetables. It all coincides.”
Bad, bad bugs
Among the “worst bugs” are strains of H. pylori that express certain bacterial virulence factors, including a group of linked genes called the “cag pathogenicity island.” Infection with strains of H. pylori that include cag genes (cag-positive strains) increases a person’s risk for severe gastritis and stomach cancer.
Peek and his colleagues are continuing to search for H. pylori factors that play a role in carcinogenesis. They are using proteomic technologies to identify the proteins expressed by different H. pylori strains – from Colombian patients with and without cancer.
They’ve also demonstrated that virulence factors interact with environmental factors, like a high-salt diet.
“Dr. Correa’s group has known for years that populations with a high-salt diet (such as the Andes population) have a higher risk for gastric cancer,” Peek says. “It could be that salt itself damages the stomach, or it might be working in synergy with H. pylori infection.”
To explore a possible interaction between salt and H. pylori, Timothy Cover, M.D., professor of Medicine at Vanderbilt, Peek and colleagues studied gene expression in cag-positive H. pylori strains exposed to high-salt conditions. They found that high salt – at levels achievable in the human stomach – increased the expression of certain virulence factors.
Keith Wilson, M.D., professor of Medicine and Cancer Biology at Vanderbilt, and his team have demonstrated that H. pylori bugs alone – without contributions from the human host – have the ability to cause cancer. They have infected gerbils with H. pylori strains from the high- and low-risk regions of Colombia, and they’ve shown that the high-risk strains cause more dysplasia (one stage in the cancer cascade) and cancer compared to the low-risk strains

“It really emphasizes why we need to learn about how H. pylori causes gastric cancer and what puts people at risk, because there are plenty of reasons not to just treat everyone who’s infected.” –Keith Wilson, M.D.
They’ve also discovered a mechanism that could explain the difference.
They previously showed that H. pylori induces expression of a protein called spermine oxidase, an enzyme whose action produces hydrogen peroxide. Hydrogen peroxide generates free radicals (oxidative stress), which can damage cellular components, including DNA.
Wilson and his colleagues found that high-risk H. pylori strains induced higher levels of spermine oxidase in cultured gastric cells and in gerbils, compared to low-risk strains.
“We think that this spermine oxidase pathway within the epithelium, in response to H. pylori, is a major source of the oxidative stress that can initiate carcinogenesis in the stomach,” Wilson says.
“It’s very exciting as a proof-of-principle that we can take the high-risk strains of H. pylori out of people, and that they cause more oxidative stress and DNA damage in cells, and more cancer in gerbils.”
Not every person colonized by high-risk or virulent strains of H. pylori gets cancer though, which suggests that other factors – such as a person’s inflammatory response to the infection – contribute to cancer risk.
Individuals who have genetic variations that cause them to produce high levels of certain pro-inflammatory molecules (IL-1beta and TNF-alpha) have a higher risk of developing gastric cancer, but only if they are infected with H. pylori, Peek explains. And if they are infected with more virulent strains, their risk of gastric cancer is increased 90-fold.
“It looks like there is a distinct interaction that’s occurring between the bacteria and the host genotypic profile that augments the risk of gastric cancer,” he says.
A complicated relationship
H. pylori‘s persistence in colonizing the human stomach, and the fact that only a small percentage of the people infected have ill effects, raises the question: is this bug doing something beneficial?
There’s intriguing evidence that infection with H. pylori may protect the esophagus – perhaps by reducing acid secretion – and may prevent the development of allergic and autoimmune diseases – perhaps by promoting certain types of immune responses.
Retrospective studies have pointed to this inverse relationship – esophageal reflux disease (Barrett’s esophagus), esophageal cancer, asthma and other allergic disorders occur more frequently in people who are not infected with H. pylori.
The former director of Vanderbilt’s Division of Infectious Diseases, Martin Blaser, M.D., now at New York University, advocates caution in our medical approaches to H. pylori, Correa says.
“He says that curing this infection is an unnatural thing – that we came out of Africa together 60,000 years ago, and we’re still together,” Correa says.
As H. pylori infection rates have fallen in countries like the United States, possibly because of increased use of antibiotics during childhood, the rates of esophageal reflux diseases, asthma and other allergic disorders have risen, Peek points out.
“It’s very intriguing to think that long term colonization with H. pylori may prevent diseases that we see escalating at alarming rates in the developed world,” he says.
We appear to have a complicated relationship with these “companions” of ours.
“It really emphasizes why we need to learn about how H. pylori causes gastric cancer and what puts people at risk, because there are plenty of reasons not to just treat everyone who’s infected,” Wilson says.
The investigators expect that their studies of H. pylori will identify concepts and mechanisms that apply broadly to inflammation-induced cancers elsewhere in the body.
“Inflammatory bowel disease has a very high risk of colon cancer – so understanding how inflammation in the colon may initiate the carcinogenic cascade is important because the incidence of those diseases is going up,” Peek says. “I think what we understand about a very defined etiologic agent – H. pylori – and a very defined disease – gastric cancer – can be broadened to other diseases that are more common and incur more of a burden on the U.S. population.”
It’s been an interesting journey since he first learned about ‘helicopters’ in his medical school days, Peek says. And he’s looking forward to the discoveries to come.
Rotor up, and away.
***
Down the hatch

In the early 1980s, physicians thought they knew what caused ulcers – it was stress.
“If you had an ulcer, you had it for life. It meant that you had to be on acid suppression, you had to not smoke, you had to eat a bland diet…and it was thought that in times of stress, your ulcers might flare,” recalls Keith Wilson, M.D., professor of Medicine and Cancer Biology at Vanderbilt University.
Barry Marshall, M.B., B.S., a clinical fellow at the time, and pathologist Robin Warren, M.B., B.S., in Perth, Australia, had another idea. They believed that bacteria were causing inflammation (gastritis) and ulcers.
Over the course of several years, Warren had noticed small curved bacteria colonizing the lower part of the stomach in about half of all biopsies he examined. And he saw signs of inflammation, always close to the bacteria.
In fact, other pathologists had reported the presence of curvy bacteria in the stomach – since the late 1800s – but the observations were ignored. Scientific dogma held that bacteria couldn’t colonize the stomach with its harsh acidity and pumping action, so the bacteria in biopsy samples were believed to be contaminants.
Marshall succeeded in growing the bacteria from tissue samples in culture, but he was unable to infect any animal models to show that the bacteria caused gastritis. Facing skepticism from the medical establishment, he opted to infect a human: himself.
He had an endoscopy procedure to show that he was not infected; then he mixed up a flask full of bacteria and guzzled it.
The rest, as they say, is history. Marshall developed severe gastritis, a precursor to ulcer disease. Although his infection appeared to spontaneously clear, Marshall treated himself with antibiotics to be sure of the bug’s elimination from his stomach.
He and other investigators were then able to demonstrate in patients that antibiotic therapy eliminated gastric ulcers.
For their discovery of Helicobacter pylori and its role in gastritis and peptic ulcer disease, Marshall and Warren received the 2005 Nobel Prize in Physiology or Medicine.
“It really blew the conventional thinking out of the water to say this one bacteria is the cause of ulcers – and oh, by the way, it’s also a cause of gastric cancer,” Wilson says. “The discovery of H. pylori was huge in terms of people not dying from ulcer disease – if you detect it and treat it, they should never have an ulcer again.”
Photos by Joe Howell
H. pylori illustration: Photograph by SPL / Photo Researchers, Inc
Flask photo: ©iStockphoto.com/Avesun
1 Comment
Sorry, the comment form is closed at this time.

Your article on Helicobacter pylori (momentum.spring10, page 11-15) was excellent!! I find the historical aspect very interesting. I can now include this in my lecture note (and acknowledge you) on peptic ulcer disease for my surgical residents.
Thanks for the information.
Oluwole G. Ajao
University College Hospital (UCH),
Ibadan,
Nigeria.
Comment by Oluwole G. AJAO — July 7, 2010 @ 9:15 am